Prof Pisit shares recent health economic data from Thailand, revealing that the GAAD score is cost-effective for HCC surveillance among Thai population
Interview transcript:
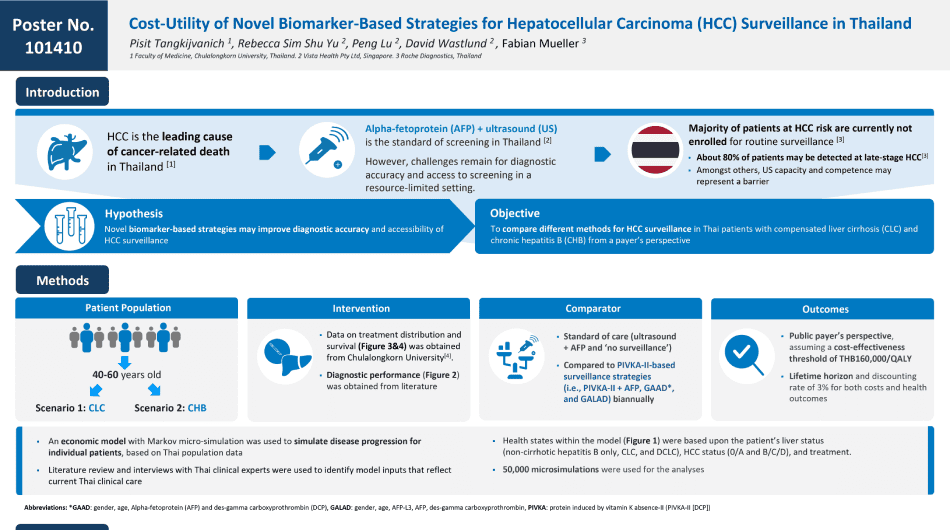
Introduction
Hello everyone. I am Dr. Pisit Tangkijvanich from the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. Nice to meet all of you.
Could you provide an overview of the HECON study?
Hepatocellular Carcinoma, or HCC is one of the most common cancers in Thailand. From Global Cancer Statistic 2020, liver cancers, especially HCC, is the number 1 of cancer in Thailand with its highest incident in men and the fourth highest incidence in women. HCC is also a leading cause of cancer mortality in Thailand, like other countries in Southeast Asia. Together, these data highlight the importance of HCC as a major public health problem in our country.
The majority of HCC occurs in patients with chronic viral hepatitis, including hepatitis B and hepatitis C, fatty liver disease, and heavy alcohol consumption. It is generally accepted that the screening or regular surveillance for HCC should be performed in patients known to be at risk of this cancer, because the surveillance could identify HCC at an early stage and can improve the overall survival of the patients receiving curative treatment, such as surgical resection or liver transplantation.
Most professional society guidelines recommend using ultrasound and serum alpha fetoprotein, or AFP every 6 months for HCC detection in at-risk patients, such as those with cirrhosis. However, ultrasound (US) is operator dependent and its sensitivity is variable between centre to centre. Moreover, the US may have lower sensitivity in patients with obesity or fatty liver disease. As a result of US limitation, more accurate and accessible (surveillance) programs that could improve HCC early detection are required.
Currently, there are several emerging strategies for HCC detection. Among them, GAAD score which is derived from Gender, Age, and the combination of double tumour markers including AFP, and DCP (or PIVKA-II), is a promising tool for early detection of HCC. The available data show that GAAD score is superior to US for HCC diagnosis with a high sensitivity and specificity.
So the aim of our HECON study was to compare cost-effectiveness analysis between GAAD score with the standard-of-care using US plus AFP for HCC surveillance in Thai patients with compensated cirrhosis and chronic hepatitis B.
Could you walk us through the methodology employed in the HECON study and discuss key findings or results?
We selected cirrhotic patients in our study because these patients are at-risk of developing HCC as the incidence rate is more than 1.5% per year. For non-cirrhotic hepatitis B, we included this group of patients because the infection is highly prevalent in Thailand and also the subgroup of patients that at-risk of HCC, especially among males older than 40 years, and females older than 50 year, or those with family history of HCC.
So, we performed an economic model with Markov micro-simulation to simulate disease progression for individual patients, based on Thai population data. Literature review and interviews with Thai clinical experts were also used to identify model inputs that reflect current Thai clinical practice. Health states within the model were based on the patient’s underlying liver disease, such as cirrhosis or non-cirrhotic hepatitis B, HCC staging, such as early or late stage and treatment outcome according to the cancer stage.
Our results showed that GAAD score was cost-effective for Thai populations at the willingness to pay threshold of 160,000 THB (or approximately 4,400 USD). In fact, GAAD yielded lower cost and a better health outcome compared to US plus AFP. In addition, sensitivity analysis confirmed that routine surveillance using GAAD score had at least 55% probability of being cost-effective compared to no surveillance.
Together, our data indicate that GAAD score is suitable for use as a screening tool in Thailand.
In your opinion, what makes the HECON data important in the context of changing healthcare policies and decision-making in Thailand?
As the burden of liver cancer is high, HCC surveillance and control must be considered a public health priority. However, in Thailand, national efforts are focused on the control of viral hepatitis, which is primary prevention for HCC rather than the cancer surveillance.
So our data show that the new method using GAAD score is cost-effective, and importantly more feasible than US plus AFP testing, because GAAD score can be done the same day in the clinics. That will help in reducing several patient barriers such as transportation or logistical concerns.
Our data could play an important part in supporting the policymakers for making the best decision within limited resource in our country, to accelerate the reimbursement program for HCC surveillance in the future.
Are there any challenges or considerations that healthcare professional should be aware of when interpreting or applying the results of the HECON study in clinical practice?
Although our study provides promising results, there might be some concerns about the role of GAAD score as a screening tool for HCC detection. For example, in countries where there are inadequate facility for cancer therapy, the benefit of early detection might be reduced as limited number of patients could achieve curative treatment. In contrast, GAAD score is more suitable in community hospital or rural areas where access to US is limited, such as some areas in Thailand and many countries in the APAC region.
How do you foresee the results of the HECON study will help to inform clinicians’ HCC surveillance and clinical practice in the APAC region?
I think the results of HECON study can be used not only in Thailand but also can be applied to other country as well, which had a similar situation as Thailand, for example, the Philippines or Vietnam. Thank you.
The views and opinions expressed by Prof. Pisit Tankijvanich are his own views and opinions. Roche disclaims all liability in relation to these views and opinions.